When boiler equipment does not meet state or federal emissions requirements, operators may require after-treatment systems. After-treatment systems contain emissions control catalysts, depending on the pollutant that needs to be controlled. Such systems can come as part of the boiler package from the manufacturer or be retrofitted to an installed unit.
There are two types of catalysts that can be incorporated into a system. For control of oxides of nitrogen (NOx), it is a selective catalytic reduction (SCR) catalyst, while, for carbon monoxide (CO), volatile organic compounds (VOCs), and formaldehyde (HCHO), it is oxidation catalyst.


Oxidation Catalysts
Oxidation catalysts create a reaction between CO, VOCs, HCHO, and atmospheric oxygen to convert pollutants to carbon dioxide (CO2) and water (H2O). They have a very large operating temperature range of 350°-1,250°F. Generally speaking, the higher the exhaust temperature, the better the conversion rates will be. These are passive components that require little to no maintenance except for washing/cleaning periodically, depending on the application and operation.

In their most common design, metal foil substrates have a pattern embossed into the foil that creates a cell pattern, which is either a herringbone or straight geometry, that is then layered with a flat piece to create the passages through which the exhaust gasses flow. The finished catalyst is encased in a stainless steel frame for installation into the after-treatment system, as shown in Figure 3. The advantages of metal substrates are their high geometric surface area and low-pressure drop associated with the thin walls. The foils in metallic substrates can be brazed/welded together to provide good mechanical durability and resistance to thermal shock. This applies to both SCR NOx catalyst and oxidation catalyst.


Several factors contribute to the effectiveness of the catalyst for each pollutant. Residence time in the catalyst, exhaust temperature, and precious metal loading (light to heavy) all have an impact on performance. The graph in Figure 4 is an example of how each of the pollutants would respond versus temperature for a particular catalyst composition.
The precious metals that facilitate the reaction used in oxidation catalysts are platinum (Pt) and palladium (Pd). These metals are also used in automotive and heavy equipment catalytic converters, the catalysts for stationary engines used in generators and the oil/gas industry, and in large gas turbine power plants. As more stringent pollution regulations are being enacted around the world, it is expected the demand for these rare metals will increase for the foreseeable future. The selection of which metal, or if a combination of them is appropriate for a boiler application, will depend upon the specific requirements the after-treatment system has to achieve.
A catalyst with more precious metal content or loading will achieve better conversion efficiencies at a lower temperature and, additionally, have a longer operational life span, as shown in Figure 5. However, as this increases the cost of the catalyst, there is always the balance between desired life, required performance, and the final price.
SCR Catalysts
SCR is the term used to describe the chemical reaction across a catalyst where harmful NOx is converted into H2O and nitrogen (N2). Unlike an oxidation catalyst, an SCR catalyst requires the use of an additional reagent (urea, diesel exhaust fluid, ammonia, etc.) to facilitate the reaction. An SCR catalyst can reduce NOx from almost any source.
As seen in Figure 6, a complete after-treatment system uses an oxidation catalyst in series with the SCR catalyst to address all the criteria pollutants that can be found in boiler exhausts.
Types of SCR Catalysts
SCR catalysts are primarily comprised from titanium oxide (TiO2), tungsten oxide (WO3), molybdenum trioxide (MO3), and vanadium pentoxide (V2O5) instead of the Pt and Pd found in oxidation catalysts. SCR catalyst technology was commercialized in the 1950s and ‘60s for control of NOx emissions from coal-fired power plants. With such a long history, a variety of catalyst configurations have been introduced, each with its own advantages and drawbacks. A brief discussion of each follow.
Ceramic Honeycomb Catalyst

The oldest form of SCR catalyst is the ceramic honeycomb style. It is made by blending the active constituents along with binder materials into a clay that is extruded to form a cellular structure. The formed catalyst is then dried and fired in a kiln. The advantage of the ceramic honeycomb is that, because it is a homogenous composition, it has the longest life span potential. This comes at the expense of being heavier and having a higher pressure drop for comparable performance relative to other styles. Ceramic catalysts are also susceptible to breakage due to the brittleness factor of the material. Ceramic SCR catalysts have an operating temperature range of 450°-950°.
There are many manufacturers of ceramic honeycomb catalysts, both domestic and foreign. This type of catalyst is used in many different applications, including boilers, coal plants, natural gas turbines, and diesel engines. The honeycomb catalyst has the longest lead time for manufacturing compared to metallic or corrugated catalysts available in today’s current market.
Corrugated Fiber Catalyst
This style is comprised from a fiber material that is corrugated and layered to create a cellular structure that is then saturated with a slurry of the active constituents before being dried and cured. This form of SCR catalyst has been especially optimized for natural gas applications. The manufacturing process is flexible to offer a number of cell sizes, which is used to balance the performance against the pressure drop. In comparison to ceramics, corrugated catalysts offer much higher activity in combination with lower pressure drop. Corrugated catalysts have a high degree of tolerance against catalyst poisons because of the catalyst structure. Due to the low weight of the corrugated catalyst, it heats up fast during startup and therefore comes into compliance more quickly than ceramic honeycomb. This catalyst type is manufactured in the U.S.
The fiber can become brittle over time while in service, which can lead to breakage if there were to be a sudden pressure/flow event, such as a backfire. Corrugated fiber SCR catalysts have an operating temperature range of 450°-900°.

Metal Foil Catalyst

SCR catalysts coated on metal foil have the same performance, pressure drop, and durability advantages as those described above for oxidation catalysts. The ruggedness of the foil makes it the best selection for applications with high vibration or other severe duty risks. The flexibility offered by the range of foil geometries means the catalyst is more readily tailored to achieve more stringent emission requirements. This can also lead to a smaller volume of catalyst in comparison to the other substrate styles being required to achieve the required performance.
Metal foil catalysts can be the most expensive style on a unit volume basis due to the cost of the foil and the more complex manufacturing process. However, if a smaller volume of catalyst is required, this drawback is mitigated. Metal foil SCR catalysts have an operating temperature range of 450°-1,125°.
Catalyst Washing
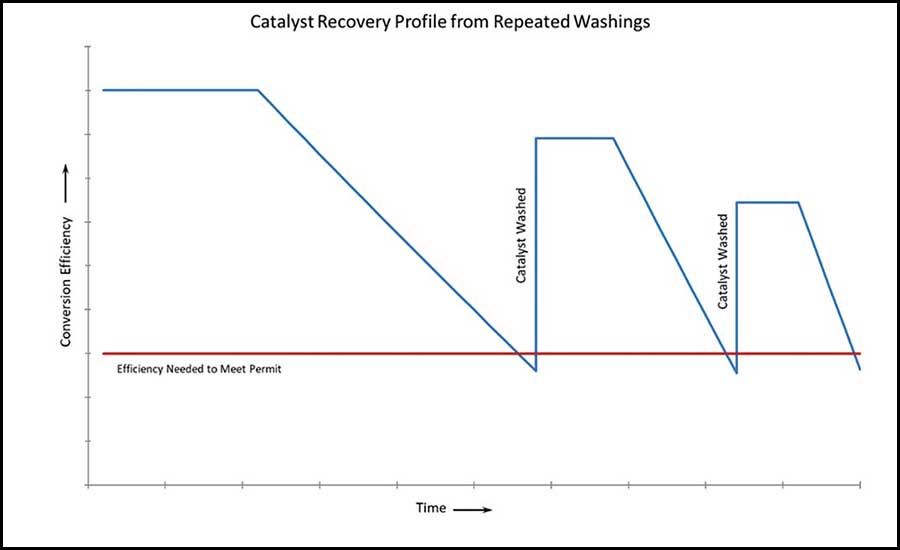
In time, all catalysts can become fouled with dirt, ash, and ingested debris. Cleaning the catalyst is a viable technique to remove much of the accumulation and extend its life span. Catalysts must be cleaned according to the manufacturer’s recommended procedure to avoid damaging the catalyst composition. Failure to do so can render the catalyst inoperable and void the warranty. One thing to bear in mind is that cleaning will not restore the catalyst to its brand-new condition. The graph in Figure 10 shows the general trend in the performance of a catalyst with repeated washings.
In Conclusion
Catalytic after-treatment systems are an established technology for protecting the air we breathe daily, which should be important to everyone. Several catalyst manufacturers have innovated their catalyst technologies based on their long history and wide-ranging experience across multiple industries.
Catalyst selection is a multifaceted design process that balances factors such as pressure drop limits, space available, warranted life, and compliance requirements to arrive at the optimum catalyst parameters. It is important to select which catalyst type works best in each application. The best practice to achieve this is to develop a technical specification and let each manufacturer propose its specific solution for your application.